COVID-19 Information
This is an evolving situation. Please continue to monitor our website so that you are kept abreast of any new developments.
Latest News
Page last updated Jan. 8, 2024.
Most Recent
COVID-19 Program Update – mRNA Bivalent Vaccines for Primary Immunization Series for All Ages as of July 24, 2023
- Monovalent mRNA vaccines will no longer be available for primary series for all ages as of July 24, 2023.
- All monovalent mRNA vaccines should be disposed of by July 23, 2023.
- Age-appropriate bivalent Omicron-containing vaccines should be administered to start or complete a COVID-19 vaccine primary series in those 6 months and older.
Extended: Non-Traditional Immunizers
The Ministry of Health has extended the approval of non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns, and pharmacist extended interns to administer publicly-funded COVID-19 and Influenza vaccines.
Please see the Ministerial Order Designating Additional Vaccine Providers for COVID-19 and Influenza Vaccine here.
These immunizers are enabled by provisions in The Public Health Act, 1994 and through SCPP emergency bylaws and are valid until March 31, 2024.
Note that only pharmacy student interns, extended interns and pharmacy technicians remain as non-traditional immunizers able to administer COVID-19 and influenza vaccines. Effective 11:59 pm on March 31, 2023, all other emergency immunizers can no longer provide immunizations unless their normal scope of practice enables it.
The following applicable documents and web page will be updated shortly:
Expired/Expiring Vaccine
The below was sent to pharmacy managers by the Drug Plan and Extended Benefits Branch.
Expiring Dec. 8, 2022:
| Vaccine | DIN | LOT | EXPIRY |
| Moderna Monovalent (red cap/light blue label) | 02510014 | 069B22A | December 8, 2022 (MUST be discarded after today) |
Expired vaccines still appearing in community pharmacy VDTS accounts:
| Vaccine | DIN | LOT | EXPIRY |
| Pfizer-BioNTech (purple cap/ purple boarder) | 02509210 | FN7934 35035BD FM2952 FM7380 FN9501 FT7276 | 2022-Oct-31 2022-Oct-31 2022-Oct-31 2022-Nov-30 2022-Nov-30 2022-Nov-30 |
Please ensure your fridges are checked, expired vaccine is discarded and inventory is updated via VDTS and submit a wastage report to the Ministry.
Cold Chain Processes
In the month of November the Ministry of Health noted an increase in the number of pharmacies submitting cold chain break reports due to human error. The fall season is a very busy time, however, it is essential that the utmost care be taken in the storage and handling of all biological products, in order to minimize wastage and protect the potency of the vaccine.
Please review the following important vaccine storage principles:
- Place new stock into the refrigerator immediately after receiving it from your distribution center.
- Keep vaccines in the refrigerator or in a cooler with cold packs until ready to administer.
- Keep all vaccines in their original boxes until they are ready to be used (protect from light, if applicable)
- Never leave vaccine outside the refrigerator (e.g., on counters or in coolers after finishing a clinic or at the end of day).
- Carefully scan you work environment frequently and at the end of each day to prevent cold chain breaks due to human error.
Thank-you for your ongoing support of the COVID-19 and Influenza Immunization Programs in Saskatchewan.
Registrar's Enactment of Part K – Extending a Drug Previously Issued by a Pharmacist
To ensure patients have continued access to their medications for chronic conditions, the SCPP Registrar is enacting section 10(5)(b) of Part K of the SCPP Regulatory Bylaws, effective immediately.
This will allow pharmacists to prescribe (extend) a prescription for a patient, when the most previous prescription for that drug was issued by a licensed pharmacist, so long as the following conditions are met:
- Patient Eligibility
Only patients with chronic diseases/conditions who are stable on their medications. This means:- Each request must be judged on an individual basis and only after considering the patient’s medical history and medication profile. The pharmacist must be satisfied that the treatment with the medication has remained relatively stable (i.e., no significant changes to dosages or drug therapy).
- Prescription Duration
Pharmacists may prescribe a maximum duration of three (3) months, and thereafter the patient must see their practitioner. This means a pharmacist may prescribe:- a 34-day supply, up to three (3) times;
- a three-month supply as a single fill; or
- a 100-day supply for a drug listed in the SK Formulary’s 100 Day List.
As per the NAPRA/SCPP Standards of Practice, pharmacists must review the information in all of the patient’s available health records, and assess the patient’s health status and unique circumstances, including an assessment of the appropriateness of therapy when prescribing (extending). Also see Prescriptive Authority – Pharmacists for other standards that apply.
As a reminder, section 2(2)(i) of Part K of the SCPP Regulatory Bylaws requires pharmacists to document their rationale for exercising this exemption on the Pharmacist Assessment Record (PAR).
This emergency exemption is being enacted in collaboration with the College of Physicians and Surgeons of Saskatchewan to help address concerns with patient access to family physicians.(See Emergency Exemptions for Prescribing Authority). It will remain in effect until Sept. 30, 2023, or until revoked by the SCPP Registrar.
Immunizing and Testing
COVID-19 Program Update – mRNA Bivalent Vaccines for Primary Immunization Series for All Ages as of July 24, 2023
- Monovalent mRNA vaccines will no longer be available for primary series for all ages as of July 24, 2023.
- All monovalent mRNA vaccines should be disposed of by July 23, 2023.
- Age-appropriate bivalent Omicron-containing vaccines should be administered to start or complete a COVID-19 vaccine primary series in those 6 months and older.
Extended: Non-Traditional Immunizers
The Ministry of Health has extended the approval of non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns, and pharmacist extended interns to administer publicly-funded COVID-19 and Influenza vaccines.
Please see the Ministerial Order Designating Additional Vaccine Providers for COVID-19 and Influenza Vaccine here.
These immunizers are enabled by provisions in The Public Health Act, 1994 and through SCPP emergency bylaws and are valid until March 31, 2024.
Note that only pharmacy student interns, extended interns and pharmacy technicians remain as non-traditional immunizers able to administer COVID-19 and influenza vaccines. Effective 11:59 pm on March 31, 2023, all other emergency immunizers can no longer provide immunizations unless their normal scope of practice enables it.
The following applicable documents and web page will be updated shortly:
COVID-19 Booster Dose Clarification and Interval Revision
Expired/Expiring Vaccine
The below was sent to pharmacy managers by the Drug Plan and Extended Benefits Branch.
Expiring Dec. 8, 2022:
| Vaccine | DIN | LOT | EXPIRY |
| Moderna Monovalent (red cap/light blue label) | 02510014 | 069B22A | December 8, 2022 (MUST be discarded after today) |
Expired vaccines still appearing in community pharmacy VDTS accounts:
| Vaccine | DIN | LOT | EXPIRY |
| Pfizer-BioNTech (purple cap/ purple boarder) | 02509210 | FN7934 35035BD FM2952 FM7380 FN9501 FT7276 | 2022-Oct-31 2022-Oct-31 2022-Oct-31 2022-Nov-30 2022-Nov-30 2022-Nov-30 |
Please ensure your fridges are checked, expired vaccine is discarded and inventory is updated via VDTS and submit a wastage report to the Ministry.
Cold Chain Processes
In the month of November the Ministry of Health noted an increase in the number of pharmacies submitting cold chain break reports due to human error. The fall season is a very busy time, however, it is essential that the utmost care be taken in the storage and handling of all biological products, in order to minimize wastage and protect the potency of the vaccine.
Please review the following important vaccine storage principles:
- Place new stock into the refrigerator immediately after receiving it from your distribution center.
- Keep vaccines in the refrigerator or in a cooler with cold packs until ready to administer.
- Keep all vaccines in their original boxes until they are ready to be used (protect from light, if applicable)
- Never leave vaccine outside the refrigerator (e.g., on counters or in coolers after finishing a clinic or at the end of day).
- Carefully scan you work environment frequently and at the end of each day to prevent cold chain breaks due to human error.
Thank-you for your ongoing support of the COVID-19 and Influenza Immunization Programs in Saskatchewan.
UPDATE: Pharmacists Prescribing Paxlovid
The Paxlovid Distribution, Prescribing and Assessment program has been extended until March 31, 2023, or at the discretion of the Ministry of Health and/or the Chief Medical Health Officer.
The expanded prescribers include community physicians, nurse practitioners and pharmacists. Pharmacists are reminded about the following conditions as set by SCPP Council:
Pharmacists who prescribe Paxlovid will be required to:
- receive training offered by CPDPP prior to prescribing – training occurred on May 12 and 13 and the sessions were recorded for ease of access.
- follow the medSask algorithms, guidelines, patient assessment record and drug interaction tools as approved by Council, and
- follow any other conditions specified by the Ministry of Health and/or Saskatchewan Health Authority for this publicly funded program.
Please see the medSask website for access to the following documents:
- Paxlovid Patient Assessment Record (PAR)
- Paxlovid Algorithm
- Paxlovid Eligibility Overview
- Paxlovid Drug Interactions Table
- Paxlovid Patient Handout
To assist members, please also see SCPP's Paxlovid Prescribing – Frequently Asked Questions for Pharmacists.
Bivalent Booster Fall Program
Expansion of Moderna Bilvalent Dose Eligibility
Provision of Moderna Spikevax® Bivalent Original/Omicron Vaccine
Vaccine Boosters for Children 5-11 Years Old
Expansion of Booster Dose Eligibility
Clarification on COVID-19 Booster Dose Intervals
Clarification on COVID-19 Booster Dose Intervals
Expansion of COVID-19 Second Booster Eligibility
Update: Non-Traditional Immunizers
The Ministry of Health has extended the approval of non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns, pharmacist extended interns, and formerly licensed pharmacists, to administer publicly-funded COVID-19 and Influenza vaccines.
Please see the Minister's Order Designating Additional Vaccine Providers for COVID-19 and Influenza Vaccine here.
These immunizers are enabled by provisions in The Public Health Act, 1994 and through SCPP emergency bylaws and are valid until March 31, 2023.
Please see the documents below for detailed guidance:
- All individuals 70 years of age and older living in Saskatchewan.
- All individuals 50 years of age and older living in First Nations.
- All individuals 50 years of age and older living in the Northern Service Administration District (NSAD) – Far North in Saskatchewan.
- Healthy individuals aged 12 years of age and older (interval applies to first booster doses).
- Adults living in a long term care home or other congregate living setting for seniors 65 years and older (interval applies to first and second booster doses).
COVID-19 Rapid Antigen Tests in Pharmacies Updates
Note the current authorizations as outlined in Saskatchewan's Chief Medical Health Officer's letter here.
Vaccine Booster Interval and Eligibility Update
Rescinded – Emergency Enactment Enabling COVID-19 Rapid Testing
Below is a recent memo sent to all practising and non-practising pharmacy professionals.
The Emergency Enactment of Part M of the Bylaws enabling COVID-19 Rapid Antigen Testing in pharmacies for proof of negative test for non-essential activities and workplace access will be rescinded effective Feb. 14, 2022, at 12:01 am.
The Government of Saskatchewan has announced it will be removing the Proof of Negative Test requirement as a COVID-19 public health measure (see here), effective Feb. 14, 2022, at 12:01 am.
This means that the SCPP’s Emergency Enactment of Part M of the Bylaws to support the public health order is no longer in effect as of Feb. 14, 2022, at 12:01 am. As such:
- Pharmacies that have submitted the SCPP Emergency Enactment Declaration are no longer eligible to register with the Ministry of Health (see here) as an Approved Rapid Antigen Testing Service Provider for proof of COVID-19 negative test (public access to non-essential businesses or work).
- Pharmacists may not perform rapid antigen tests on the public for proof of negative test for public access to non-essential activities or for workplace requirements.
- Pharmacists may continue to provide COVID-19 rapid antigen testing for travel purposes only under the SCPP terms and conditions specified (see Emergency Enactment – COVID-19 Rapid Antigen Testing in Pharmacies) and until the end of the current public health order (see here), which is in effect until Feb. 28, 2022.
- Pharmacists are authorized to perform Health Canada approved COVID-19 rapid antigen tests as part of an Occupational Health and Safety/workplace screening initiative (see COVID-19 Occupational Health & Safety Testing in Community Pharmacies (“Test to Protect”)); and
- Pharmacies are authorized to sell Health Canada approved COVID-19 self-tests to the general public for personal use and distribute Health Canada approved COVID-19 rapid antigen point-of-care tests under a provincial or federal program. (See SCPP memo sent Jan. 24, and see Sale and Distribution of Medical Testing Devices and Other Diagnostic Products for more information about selling and distribution requirements.)
For an overview of current measures see Status Update – COVID-19 Rapid Antigen Tests in Pharmacies.
Part M of the Regulatory Bylaws – Status Quo
Once the public health order is removed, the emergency bylaws for Part M are no longer active and the status quo resumes.
As part of their regular scope of practice, pharmacists are permitted to perform laboratory tests (which includes point of care testing) for the purposes of drug therapy management as long as they meet the qualifications, terms, conditions, and standards set out under The Pharmacy and Pharmacy Disciplines Act, under The Medical Laboratory Licensing Act, under Part M of the SCPP Regulatory Bylaws, and the following reference documents:
- Laboratory Tests and Medical Devices – Accessing, Ordering, Performing, Using, or Interpreting;
- Performing Tests for Drug Therapy Management; and
- Sale and Distribution of Medical Testing Devices and Other Diagnostic Products
As announced in the SCPP memo on Jan. 24 (see here), these reference documents have been updated and new Council policies have been adopted to support pharmacists practising to their full scope. Further information on these updates will be provided in our next edition of MicroSCOPe.
COVID-19 Booster Doses for 12-17
Please see the notice here.
Emergency Enactment COVID-19 Rapid Antigen Tests in Pharmacies
SCPP Council held a meeting on Jan. 21, 2022, to continue discussions and review feedback with respect to rapid antigen testing within pharmacies.
Council has approved the enactment of emergency bylaws to Part M to enable COVID-19 rapid antigen testing to occur within pharmacies effective Jan. 21, 2022, and to align with the expiration of the provincial public health order. As such, Council policy has now been finalized – please see Emergency Enactment Rapid Antigen Tests in Pharmacies which includes frequently asked questions.
Amendment to COVID-19 Vaccine Booster Dose Program
COVID Rapid Tests in Pharmacies
Below is a recent memo sent to all practising and non-practising pharmacy professionals.
The College has received numerous questions regarding selling, distributing, and performing rapid antigen tests through the pharmacy given the various federal and provincial programs.
The pandemic landscape has been shifting rapidly and changes have been made to the SCPP bylaws, provincial regulations, and federal exemptions to help respond to the demands and challenges. The following is a summary of what is currently permitted (Also see Status Update – COVID-19 Rapid Antigen Tests in Pharmacies for more information).
Selling Rapid Tests to the Public
Health Canada has approved several rapid antigen tests for “self-testing” by the general public (see here). These tests may be sold to the general public; however, members would need to inform the public when they can access tests at no extra cost through federal or provincial publicly-funded programs.
Distributing Rapid Tests to the Public (Federal/Provincial Programs)
A recent Health Canada exemption (see Dec. 13, 2021 bulletin) has permitted the distribution of point-of-care rapid tests to the general public regardless of the intended end user. While no exemption is needed to distribute “self-testing devices” for use by the general public, this exemption permits “point-of-care tests” (POCT) to be distributed to the public for personal use. POCTs are designed for use by an approved operator (i.e., health care providers).
Health Canada has listed specific conditions that must be met if participating in the distribution of federal or provincially supplied rapid antigen tests
As a result, pharmacies are now able to distribute rapid tests offered through Saskatchewan’s provincial program “Rapid Antigen Self-Testing at Home” (see here) and any other federal/provincial programs under development so long as they meet the terms and conditions of the respective programs.
(Note: these exemptions are in effect until March 31, 2022, and also apply to workplace screening initiatives, see below.)
Workplace Screening/Occupational Health and Safety Testing (Federal/Provincial Programs)
Workplace screening initiatives are being administered in collaboration between federal and provincial governments. Health Canada’s Dec. 13, 2021, bulletin, expands on the previous exemption issued on June 15, 2021 (see here) in support of workplace screening.
In addition to the terms and conditions above, it also confirms that pharmacies are permitted to distribute rapid antigen tests to small and medium-sized businesses without a Medical Device Establishment Licence (MDEL).
Note: On May 10, 2021, the Registrar enacted emergency provisions under the SCPP bylaws that permitted pharmacists to perform rapid tests with specific terms and conditions, as part of federal/provincial occupational health and safety initiatives. This enactment is still in force. See COVID-19 Occupational Health & Safety Testing in Community Pharmacies (“Test to Protect”).
To ensure that clear guidance and direction are available to pharmacy professionals, pharmacy managers, and proprietors, the SCPP has created a new document and updated its three Reference Manual documents related to laboratory practices outlined in Part M of the Regulatory Bylaws:
- Laboratory Tests and Medical Devices (Accessing, Ordering, Performing, Using, or Interpreting)
- Laboratory Tests – Performing Tests for Drug Therapy Management
- Laboratory Tests – Sale and Distribution of Medical Testing Devices and Other Diagnostic Products
- Status Update - COVID-19 Rapid Antigen Tests in Pharmacies
For details on the updates to these documents, please see the updated Laboratory Tests web page. Questions for the SCPP can be directed to info@saskpharm.ca.
SCPP can provide answers to questions specifically related to the bylaws and standards; however, all other questions regarding programs should be directed to the organization delivering the program.
Additional COVID-19 Vaccine Booster Doses to Select Populations
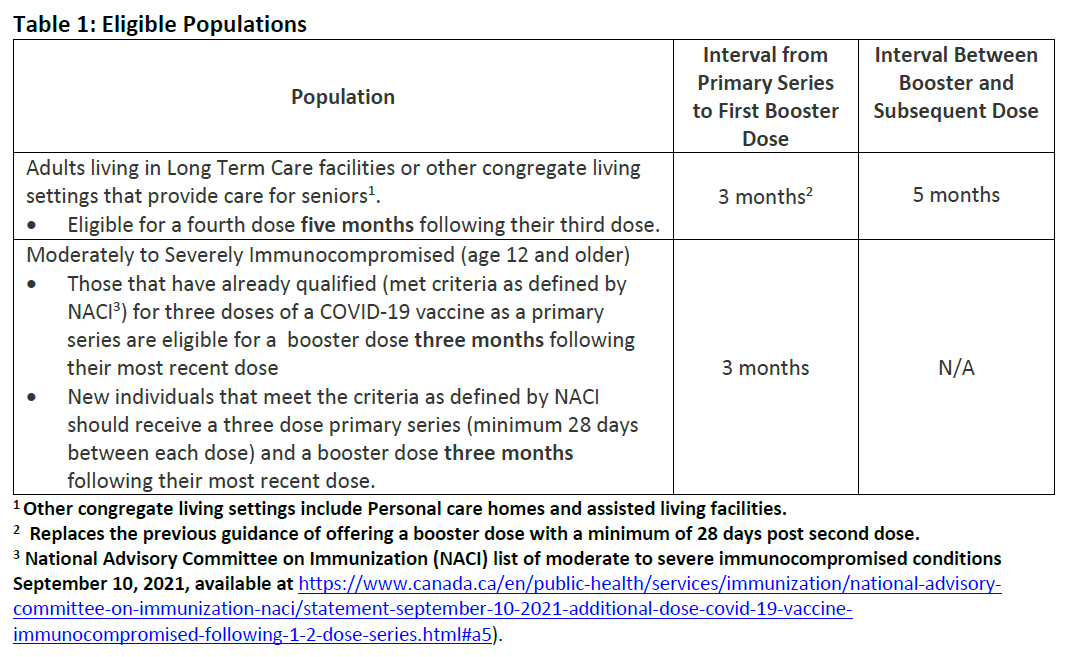
Additional COVID-19 Immunizations Discontinued for Travel Purposes
COVID-19 Vaccine Expanded Booster Information
The Government of Saskatchewan continues with booster dose expansion.
Effective immediately, the following individuals are eligible to receive third/booster doses as noted below, see the full announcement here
Populations Eligible for Booster Doses Immediately:
- Individuals aged 50 years and older
- All health care workers
- Individuals 18 years and older living in the far north and those living on First Nation communities
- Individuals born in 2009 or earlier with underlying health conditions that are clinically extremely vulnerable, including those with diabetes
Effective immediately for all booster target populations the recommended interval between second doses and booster doses is five months instead of six months.
COVID-19 Vaccine Booster Information and Additional Information
The Government of Saskatchewan has provided information on booster dose expansion and clarification, three dose primary series for immune compromised individuals, and pediatric vaccine clarification.
Effective immediately, the following individuals are eligible to receive third/booster doses as noted below, see the full announcement here
Those living in:
Immunizations for Five- to 11-year-olds
The Ministry of Health has advised that with the Health Canada approval of the Pfizer COVID-19 vaccine for children aged five- to 11-years-old, immunizations for this age group will begin. See the announcement here.
Vaccinations for five- to 11-year-olds will be available at a wide variety of locations including participating pharmacies, Saskatchewan Health Authority (SHA) walk-in clinics, mobile clinics, at schools, and at venues with easy community access near schools.
The Pfizer pediatric vaccine is a slightly different formulation with smaller doses of vaccine and immunizers will need to complete the applicable SHA training online to ensure they are competent to provide pediatric immunizations.
Please see the following helpful resources:
Johnson & Johnson Vaccinations Available at SHA Clinics
The Government of Saskatchewan issued a news release announcing that a limited supply of Janssen COVID-19 vaccines will arrive in the province for administration through a number of Saskatchewan Health Authority (SHA) clinics, effective Nov. 17, 2021.
See the news release here.
- Recipients of the single-dose Janssen vaccine are eligible for an mRNA vaccine booster dose two months after their vaccine (for all recipients, 18 years and older).
- Pharmacies can administer either Pfizer or Moderna to patients two months after their single dose of Janssen vaccine.
Non-Traditional Immunizers for Influenza Immunization Program
The Ministry of Health has approved non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns and extended interns, and formerly licensed pharmacists, to administer publicly-funded influenza vaccines. Please see the Order of Council notice here. For the complete list of non-traditional Influenza immunizers, please click here.
These immunizers are enabled by the emergency provisions to the Disease Control Regulations and through SCPP emergency bylaws and are valid until March 31, 2022.
Please see the updated Influenza Season Preparation poster as well as the Immunizer FAQs.
Reminder about Vaccine Fraud
The College would like to make members aware that falsifying vaccination records is an offence covered under Canada’s Criminal Code. Members must report any suspected vaccine fraud to the Saskatchewan Public Safety Agency at 1-855-559-5502.
The Pharmacy and Pharmacy Disciplines Act and the Code of Ethics require members to report any professional misconduct directly to SCPP, where the report will be followed up using the College’s complaint and investigative process.
Q-and-A Booster Shots for Health Care Workers
The below was communicated by the Drug Plan and Extended Benefits Branch on Oct. 21, 2021.
The following Q-and-As provide further clarification on the eligibility of health care workers for a COVID-19 vaccine booster dose effective Oct. 25, 2021.
Q: What identification will health care workers be required to provide in order to receive their boosters?
Health care workers will need to present one of the following: a copy of their licence from their professional licensing body, OR a workplace pay stub at the point of immunization OR, if they are an SHA employee, their Saskatchewan Health Authority staff identification to demonstrate that they are health care providers. It’s vital that they are at least six months from their second dose, so checking their vaccination record is key for eligibility.
Q: Is it all health care workers? In previous immunization roll-out phases, we were provided a list of occupation types.
YES, this is for ALL health care workers regardless of position. Proof of employment OR the credentials of a licensing body, in addition to being six months from their second dose, will be the only proofs required to qualify for a third dose at this time. Cross-referencing workers by occupation and determining the type of documentation that would be required has been identified as a barrier to immunization.
Please see the directive from Saskatchewan's Chief Medical Health Officer.
Expanding Booster Dose Eligibility
Starting Oct. 25, 2021, the COVID-19 vaccination booster program will be expanded. COVID-19 boosters will be administered at least six months after the second dose was received for the following groups:
- Individuals aged 65 years and older.
- Individuals living in the Far North and those living on First Nation communities, aged 50 years and older.
- Health care workers, who will be asked to present a copy of their license from their professional licensing body or a workplace pay stub at the point of immunization. Saskatchewan Health Authority staff will be required to present their staff identification.
- Individuals born in 2009 or earlier with underlying health conditions that are clinically extremely vulnerable including:
- People with severe respiratory conditions including all cystic fibrosis, severe asthma, and severe chronic obstructive pulmonary disease (COPD)
- People with rare diseases that significantly increase the risk of infections, such as homozygous sickle cell disease
- People who had their spleen removed
- Adults with very significant developmental disabilities that increase risk, such as Down's Syndrome
- Adults on dialysis or with chronic kidney disease (stage 5)
- Significant neuromuscular conditions requiring respiratory support
For more details, see the news release.
Acceptable Proof of Vaccination
As per the recent news release from the Ministry of Health, immunization records printed by the pharmacy from the eHR Viewer are not accepted as valid proof of vaccination.
The following are the accepted options for proof of vaccination.
- QR code/MySaskHealthRecord vaccine certificate either printed or on the patron's mobile device as a screenshot or in SK Vax Wallet;
- A printed hard copy of MySaskHealthRecord vaccine certificate with or without a QR code;
- Wallet cards issued at the time of vaccinations; and
- A COVID-19 vaccination printout from Saskatchewan Health Authority (SHA) public health.
See the media release for more details.
COVID-19 Vaccine Contraindications Document Updated
Expansion of COVID Self-Testing
Expanded Booster Dose Eligibility
A complete list of eligible individuals is available at Saskatchewan.ca/covid-19.
If a pharmacy wishes to increase its COVID-19 vaccine allocation for the week of Oct. 4 (delivery on Oct. 6), they need to submit those requests by 12:00 pm, Wednesday, Sept. 29 by email to DPEBimmunizations@health.gov.sk.ca.
Automated COVID-19 test results available in Saskatchewan
With the increase of positive COVID-19 cases across the province, the Saskatchewan Health Authority has moved all initial notifications for COVID test results to an automated service. In May 2021, the SHA launched the auto-notification for negative COVID-19 test results. Now, beginning on September 17, anyone tested for COVID-19 in Saskatchewan will be quickly informed of both their positive and negative COVID-19 lab test results through text message or voice call message notifications.
For more information about automated COVID-19 test results, please visit saskatchewan.ca/COVID19. For the full announcement, please click here.
Providing Immunization Records
UPDATED: Please see more recent news regarding acceptable immunization records from the Ministry of Health.
People requesting COVID-19 immunization records for travelling outside of Canada or other reasons are encouraged to sign up for MySaskHealthRecord. Instructions for downloading immunization records can be found here and there are also frequently asked questions available. However, some patients may request COVID-19 immunization records from their pharmacists who should be mindful of the following:
- Providing immunization records is similar to providing a profile for a patient, as it is a medication administration record.
- Privacy, consent, and documentation standards apply - pharmacists will need to ensure there is documented consent that the patient agreed to the service. See Reference Manual documents Privacy and Disclosure – Release of Confidential Records and Release of Confidential Records of Minors.
- Children aged 15 and older have their own MySaskHealthRecord (MSHR) and can choose whether parents have access, therefore pharmacists will need to follow privacy and consent legislation should a person other than the minor request the documentation.
- The immunization record the pharmacy prints out may not look as official or complete as the form patients can generate using their MSHR account and therefore may not be in a form accepted by the third party.
- Pharmacies will need to make the business decision whether they have the capacity to offer this service.
- There are alternatives for the public to request access to their records if they do not have computer/phone or internet access.
SCPP strongly encourages pharmacy privacy officers, in conjunction with the pharmacy manager, to have a proper written policy and procedure on this process and ensure staff are appropriately trained.
Provision of COVID-19 Vaccine Booster Doses to Select Populations
.png)
Pfizer Extended Expiry for Vaccine in ULT
The Ministry of Health has advised that Pfizer-BioNTech has extended the shelf life only for vaccine stored at ULT temperatures by three months. More information below:
- As per this notification, Pfizer-BioNTech has extended the ULT shelf-life of their COVID-19 vaccine by three (3) months.
- This includes product already in country, and product that will be arriving in the future.
- Expiry dates on all packaging, from August 2021 to February 2022, is extended by three (3) months (see table below).
- All sites will have to keep track of the updated expiry dates themselves.
- The manufacturer will not be updating their labels.
- The extended shelf life only applied to vaccine stored at ULT temperatures. The product monograph remains unchanged related to storage/transport at frozen (-20*C), fridge (2 to 8*C), and room temperature (up to 25*C)
- Pfizer-BioNTech has also extended the ULT temperature storage parameters.
- Vials can now be stored/transported between -90C and -60C.
- The Ministry’s cold chain work standard will be updated as soon as possible to reflect the changes.
- The product monograph at cvdvaccine.ca will be updated shortly - see table below from page 22.
| Printed Expiry Date |
| Updated Expiry Date |
| August 2021 | > | November 2021 |
| September 2021 | > | December 2021 |
| October 2021 | > | January 2022 |
| November 2021 | > | February 2022 |
| December 2021 | > | March 2022 |
| January 2022 | > | April 2022 |
| February 2022 | > | May 2022 |
Vaccines for Ages 12-17
While Health Canada has approved the Moderna COVID-19 vaccine for children aged 12 and over, immunizers in Saskatchewan are not authorized to administer Moderna to those aged 12-17 until authorization is provided by the Ministry of Health. Please check the COVID-19 page as well as the SCPP newsfeed regularly for any updates.
Pfizer Vaccine Approved for Children Born in 2009
Updated Pfizer COVID-19 Vaccine Fact Sheet and After-Care Sheet
Travelling and Immunization Records
One resource pharmacists can recommend to travellers is the Government of Canada webpage Use ArriveCAN to enter Canada. Travellers should be encouraged to use reliable sources for requirements to enter their destination country.
Prescribing and Drugs
Paxlovid – Expiry Extension to 18 months
Health Canada has issued a Notice of Compliance (NOC) for the shelf-life extension of PAXLOVID (nirmatrelvir and ritonavir) from 12 months to 18 months for multiple LOT#s of the Standard Dose Pack (DIN: 02524031) and the Moderate Renal Impairment Dose Pack (DIN: 02527804). Pharmacy managers were sent the below information:
- Blisters and cartons with an expiry date of August 2022 printed on the label may remain in use for an additional 9 months beyond the printed date.
- Blisters and cartons with an expiry date of January 2023 through May 2023 printed on the label may remain in use for an additional 6 months beyond the printed date.
- The approved storage conditions remain unchanged.
- The expiration date of Paxlovid Tablets must be verified prior to dispensing.
As required by the NAPRA Standards of Practice (see 1.3 and 3.1 here), pharmacy professionals must verify the accuracy of dispensed prescriptions and communicate with patients effectively. Pharmacy professionals must consider the following actions when dispensing the impacted lot# of Paxlovid®:
- Blacking out the expiry dates on the external box and each blister card, and applying a label showing the new expiration date;
- Verifying the expiration date prior to dispensing; and
- Reviewing the modifications with the patient to reassure them it is safe to use.
Affected LOT#s and extended expiry dates: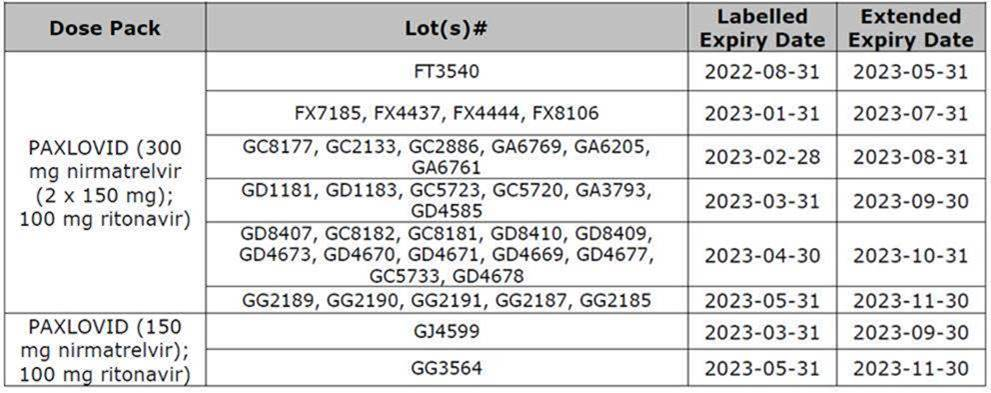
Please see the Pfizer letter for complete details.
If you have any questions, please contact DPEBImmunizations@health.gov.sk.ca.
Registrar's Enactment of Part K – Extending a Drug Previously Issued by a Pharmacist
To ensure patients have continued access to their medications for chronic conditions, the SCPP Registrar is enacting section 10(5)(b) of Part K of the SCPP Regulatory Bylaws, effective immediately.
This will allow pharmacists to prescribe (extend) a prescription for a patient, when the most previous prescription for that drug was issued by a licensed pharmacist, so long as the following conditions are met:
- Patient Eligibility
Only patients with chronic diseases/conditions who are stable on their medications. This means:- Each request must be judged on an individual basis and only after considering the patient’s medical history and medication profile. The pharmacist must be satisfied that the treatment with the medication has remained relatively stable (i.e., no significant changes to dosages or drug therapy).
- Prescription Duration
Pharmacists may prescribe a maximum duration of three (3) months, and thereafter the patient must see their practitioner. This means a pharmacist may prescribe:- a 34-day supply, up to three (3) times;
- a three-month supply as a single fill; or
- a 100-day supply for a drug listed in the SK Formulary’s 100 Day List.
As per the NAPRA/SCPP Standards of Practice, pharmacists must review the information in all of the patient’s available health records, and assess the patient’s health status and unique circumstances, including an assessment of the appropriateness of therapy when prescribing (extending). Also see Prescriptive Authority – Pharmacists for other standards that apply.
As a reminder, section 2(2)(i) of Part K of the SCPP Regulatory Bylaws requires pharmacists to document their rationale for exercising this exemption on the Pharmacist Assessment Record (PAR).
This emergency exemption is being enacted in collaboration with the College of Physicians and Surgeons of Saskatchewan to help address concerns with patient access to family physicians.(See Emergency Exemptions for Prescribing Authority). It will remain in effect until Sept. 30, 2023, or until revoked by the SCPP Registrar.
Pharmacists Prescribing Paxlovid
The Ministry of Health is expanding access to Paxlovid by increasing the eligibility criteria and expanding who may prescribe for this publicly funded drug. The Paxlovid Distribution, Prescribing and Assessment program officially launches on May 19, 2022 and is in effect until Oct. 31, 2022 or at the discretion of the Ministry of Health and/or the Chief Medical Health Officer.
The expanded prescribers include community physicians, nurse practitioners and pharmacists. SCPP Council met on May 2, 2022, and approved pharmacists prescribing for Paxlovid for the treatment of mild to moderate COVID-19 with the following conditions.
Pharmacists who prescribe Paxlovid will be required to:
- receive training offered by CPDPP prior to prescribing – training occurred on May 12 and 13 and the sessions were recorded for ease of access.
- follow the medSask algorithms, guidelines, patient assessment record and drug interaction tools as approved by Council, and
- follow any other conditions specified by the Ministry of Health and/or Saskatchewan Health Authority for this publicly funded program.
Please see the medSask website for access to the following documents:
- Paxlovid Patient Assessment Record (PAR)
- Paxlovid Algorithm
- Paxlovid Eligibility Overview
- Paxlovid Drug Interactions Table
- Paxlovid Patient Handout
To assist members, please also see SCPP's Paxlovid Prescribing – Frequently Asked Questions for Pharmacists.
Joint Statement - Section 56 Exemption Extension
Joint Statement - Ivermectin in the Prevention and Treatment of COVID-19
The Saskatchewan College of Pharmacy, the College of Physicians and Surgeons of Saskatchewan, the Saskatchewan Registered Nurses Association, the Saskatchewan Medical Association, and the Pharmacy Association of Saskatchewan have released a joint statement on Ivermectin in the Prevention and Treatment of COVID-19. Please see it here.
Registrations Open for CPDPP Injection Training Workshops
For more information on this and other requirements for COVID-19 Immunizers, please see SCPP's Training and Development page.
PIP Prescribing for COVID-19 Positive Patients
The Saskatchewan Health Authority, in collaboration with eHealth, the Drug Plan and Extended Benefits Branch and the Ministry of Health, is recommending the addition of COVID-19-specific information when prescribing electronically using the Pharmaceutical Information Program (PIP). Please see this memo for more information.
This will allow the community pharmacy to coordinate with the patient to ensure safe dispensing of medications while limiting the risk of community exposure.
Section 56 Exemption Extended by Health Canada
The current Health Canada section 56 exemption has been extended from September 30, 2020 to September 30, 2021.
The exemption, which outlines the terms for patients, and practitioners and pharmacists prescribing and providing controlled substances during the COVID-19 pandemic, has not changed. Also note that Health Canada can amend, terminate or extend the exemption if it deems it necessary to protect public health and safety.
Please see the Health Canada notice here.
Health Canada: Be a Good Steward of Medication
Health Canada advises Canadians to help support the continued supply of medications.
The COVID-19 pandemic has resulted in significant shifts in the supply and demand of certain drugs. While supply levels may be stabilizing, supply pressures continue for certain drugs. Governments, industry, pharmacy organizations, and other health sector partners continue to work together to address supply issues, and are taking measures to conserve the supply of critical drugs. Health Canada has been monitoring the supply situation closely and will take any necessary actions, in collaboration with its partners, to help ensure the continued supply of medications for Canadians.
Patients are advised not to buy more medication than required. This will help ensure that all Canadians continue to have access to the medications they need and to prevent drug shortages.
For the full advisory, please click here.
Province Lifts Limit on Filling of Prescriptions
Effective May 20th, the Saskatchewan government is lifting the supply limits on prescription medications that were introduced to guard against shortages during the COVID-19 pandemic. Though the majority of the Canadian drug supply is more stable, some drugs (e.g. salbutamol inhalers) and drug classes (e.g. sedatives and antibiotics) are in short supply. For these medications, pharmacists will use their judgement and dispense appropriate quantities. The medications supply is being actively monitored at federal and provincial levels, as the pandemic evolves.
Please see the news release here and the letter sent to pharmacy professionals here.
Pharmacist-Administered Injections During COVID-19
CPSS UDS Criteria During COVID-19
See the criteria here.
Updated Requirements for Post-Consumer Returns
Prescriptions Direct from PIP
Please see the notice to physicians and surgeons here.
Electronic prescriptions sent to the PIP system are available to any pharmacy within Saskatchewan and all pharmacists licensed in Saskatchewan have access to the PIP. It is the patient's choice as to where to have the prescription filled. This information is to alert members that you may see an increase in prescribing in the PIP.
When requested by the patient, prescriptions on the PIP should be printed and then downloaded into the local vendor software. It is important that the popup blocker on the internet browser in the pharmacy be turned off to allow for successful printing.
The download process from the integrated PIP profile is like that of a typical prescription transfer download into the local pharmacy software. Please refer to your vendor for support if needing further assistance. Downloading an electronic prescription is recommended versus creating a new prescription locally as it prevents duplicate prescriptions from appearing in the PIP.
For further assistance regarding the PIP, contact their service desk:
Toll Free: 1-888-316-7446
Fax: 1-306-781-8480
Email: servicedesk@eHealthSask.ca.
Stay the Course with 30-day Prescription Refills (CAPDM)
See the letter from Dan Chiasson, president and CEO, CAPDM, to the National Association of Pharmacy Regulatory Authorities (NAPRA).
Prescription Regulations Summary Chart Temporarily Amended
Veterinary Drugs: VASR Deadline Extension to June 30
For questions about reporting requirements, email the Veterinary Drugs Directorate or visit the Veterinary Antimicrobial Sales Reporting web page.
Continuation of Therapy when last Prescribed by a Pharmacist
Pharmacists must be familiar with the OAT guidance memo emailed on March 25, 2020 when extending such therapies.
See SCPP’s Bylaw Interpretation for Exemptions to Prescribing Authority for more details. Also see, the Practice Changes for Community Pharmacy During COVID-19 Pandemic document for a summary of current exemptions and restrictions, and for examples of situations where these changes may apply.
Please stay tuned for upcoming information from DPEBB with respect to guidance on billing for Prescriptive Authority Fees.
Dispensing Quantity Restrictions
- quantities that exceed the limits in the bylaws;
- when the most previous prescription for that drug was issued by a licensed pharmacist; or
- when no active relationship exists between a practitioner and the patient.
This emergency exemption has been enacted in collaboration with the CPSS, SRNA, SHA, and the Ministry of Health, to support health system efforts to address the COVID-19 pandemic. It will remain in effect until September 30, 2020 or until revoked by the SCPP Registrar.
In addition to this exemption, pharmacists should review and follow the Health Canada Exemption 56 letter and guidance memo from SCPP emailed on March 23, 2020 for all drugs found in the schedules of the CDSA.
Pharmacists must document reasons for exercising these exemptions on the prescription or patient’s profile and when applicable complete a PAR.
Exemptions to Opioid Agonist Therapy “OAT” Standards
Please see the OAT Emergency Contingency Plan from the College of Physicians and Surgeons of Saskatchewan.
Methadone and Witnessed Dosing and Take-home Doses
To implement these exemptions in accordance with provincial and federal requirements, the SCPP has been collaborating with Health Canada, College of Physicians and Surgeons of Saskatchewan (CPSS), Saskatchewan Registered Nurses Association (SRNA), College of Dental Surgeons of Saskatchewan (CDSS), Ministry of Health (MoH), Non-Insured Health Benefits (NIHB) and Pharmacists Association of Saskatchewan (PAS).
When is the exemption in effect:
Although enabled by Health Canada, this exemption will come into force upon official notice from the SCPP, and expires on the earliest of the following dates:
- September 30, 2020;
- The date that it is replaced by another exemption; or
- The date on which it is revoked.
What does the exemption cover:
This exemption will permit:
- pharmacists to extend prescriptions;
- pharmacists to transfer prescriptions to other pharmacists;
- prescribers (i.e. physicians, nurse practitioners, dentists) to issue verbal orders (i.e. over the phone) to extend or refill a prescription; and
- pharmacy employees to deliver prescriptions of all CDSA drugs to patients’ homes or other locations where they may be (i.e. self-isolating).
Impact on Prescription Review Program (PRP)
The Ministry of Health and all of the partners in the PRP have agreed to temporarily suspended the requirement that all PRP drugs be prescribed in writing. This means that all prescriptions for any CDSA drug and those PRP Schedule I drugs can be verbally ordered by an authorized practitioner directly to a licensed pharmacist.
The SCPP is developing resources to ensure that members are aware of expectations and requirements, and to address barriers to patient care while still protecting the public of controlled substances.
We extend a huge thank you to all of our partners for working together to remove as many barriers as possible to ensure the patients of Saskatchewan can rely on the healthcare system to meet their health care needs.
Number and Quantity of Prescription Drugs Limits
Effective immediately, Saskatchewan pharmacists may only provide:
- A ONE MONTH supply (to a maximum of a 35 day supply) in a 28 day period for all drugs not on the Maintenance Drug Schedule;
- A TWO MONTH supply in a 55 day period for drugs on the Two Month Drug List;
- A 100 Day supply in a 95 day period for drugs on the 100 Day List.
Details from the Ministry of Health can be found in the Pharmacy Information Bulletin No. 722 and the letter from the Deputy Minister of Heath to Saskatchewan pharmacists.
Posters for pharmacies to print and communicate this change can be found here:
Document: Pharmacy Poster
Document: Protect Yourself and Others - Stop the Spread of Viruses
Pharmacy Operations
Masks, the Way Forward
With the provincial masking mandates ending at 12:01 am on Monday, Feb. 28, pharmacies will need to determine their own policies with respect to masking should they choose to do so. For example, will customers/patients be required to mask in their pharmacies; whether staff will be required to wear masks; and/or whether patients will be required to wear masks when receiving particular services.
Managers and owners must also ensure Occupational Health and Safety practices are in place to ensure the ongoing safety and wellbeing of employees and patients. Further information on the provincial occupational health and public safety requirements is available at Information for Businesses and Workers or Information for Health Care Providers. In addition, the following information may be helpful as pharmacies navigate this change in provincial policy:
- SCOPe October 2020 article: Mask Use by the Public
- SCPP Reference Manual: Infection Control Standards and Guidelines
The following guidance is relevant:
- For businesses, the Government of Saskatchewan has stated the following:
“It is the choice of an individual business or facility to implement their own masking policy. If you enter a facility that requires a mask, patrons must respect the decision of the business and either comply or choose not to visit the establishment.”
“Municipalities, businesses, workplaces and event organizers may choose to continue requiring proof of vaccination or negative testing even without the public health order.” - Note that pharmacists have an ethical obligation to provide continuity of care to patients, an obligation not to discriminate against patients who have a medical condition, and an obligation to provide continuity of care to their existing patients.
- Pfizer-BioNTech vaccine – youth and adults 12 years of age or older
- Moderna vaccine – adults 18 years of age or older
- AstraZeneca vaccine – adults 18 years of age and older
- COVISHIELD vaccine – adults 18 years of age and older
- Janssen vaccine – adults 18 years of age and older
Drugs that may be administered by a licensed pharmacist with Advanced Method Certification
9 A licensed pharmacist with Advanced Method Certification may administer any of the following drugs: (a) a publicly funded vaccine provided under a provincial immunization program, where the Ministry of Health has approved administration by licensed pharmacists;
Should other COVID-19 vaccines be approved, additional authorization will be required. To read the letter from the Ministry, please click here.
Developing an Exposure Control Plan
In order to limit exposure to COVID-19, all employers are required to develop and implement an exposure control plan for their workplace. Section 85 of the Government of Saskatchewan’s Occupational Health and Safety Regulations specifies the informational elements that must be included in your exposure control plan.
These elements are outlined here.
Emergency Licencees no Longer Required
With the curve of COVID-19 in the province flattening, the Saskatchewan Health Authority has reported that the health care system is managing with the current number of cases. Therefore, SCPP will no longer be requesting or processing applications for Emergency Practising Licences. Current emergency licences will remain valid for the 90-day period.
SCPP wishes to thank all those who applied for their willingness to answer the call for help.
Reminder: AMC and First Aid / CPR Requirements
In order to provide medications by injection and other routes, SCPP requires that you maintain current First Aid and CPR Level C certification, complete all required training available through Continuing Professional Development for Pharmacy Professionals (CPDPP) and properly declare AMC during licence renewal.
Due to the COVID-19 pandemic and resulting physical distancing requirements, we have learned that in-person courses previously available to recertify for First Aid and CPR Level C are being postponed. As a result, some providers are extending their certificate expiry dates. The onus is on the member to determine if their First Aid and CPR Level C training is expired, and/or if any extension to the expiration date is being provided by the training agency. If your First Aid and CPR Level C certification is expired and you are unable to obtain proof that your certification has been extended, you should not provide medications by injection until you can re-certify.
SCPP is monitoring the situation regarding First Aid and CPR Level C training and will provide more information as it becomes available, but we encourage all members to check with their training provider.
In the March edition of SCOPe, SCPP reported in error that all CPR and First Aid certificates had been extended until September 30, 2020. It has since been confirmed that certificates from certain providers may be extended 90 days. Again, please check with your service provider.
Please find more information on the Registration page.
Joint Statement of Professional Accountability
Virtual Care
Pexip is a videoconferencing software system that is compliant with HIPA and PIPEDA, and has been approved by the Ministry of Health, eHealth and the Saskatchewan Health Authority for use on computers and mobile devices. Proper knowledge of the software is required prior to using, including how to lock a room to ensure patient confidentiality.
Although pharmacy team members already use the telephone to communicate with patients and other health care providers, videoconferencing is another tool that may help to provide services. The College, through the support of medSask, has completed a comprehensive review of the listed minor ailments and has determined that a telephone consultation where deemed appropriate by the pharmacist and the use of the Pexip virtual care system would be an acceptable alternative to in-person consultations and assessments during COVID-19. Other patient care services where Pexip may be utilized would be for SMAPs, PACT and any counselling or patient monitoring activities as required.
As always, it is the discretion of the prescribing pharmacist to ensure all standards of care are met for a telephone or virtual consultation as they would be for an in-person consultation. Meaning, each patient must receive the same high standard of care whether they are receiving that care in person, over the phone or via a virtual visit.
For more information on covered services and how to register for Pexip, click here.
To access the Pexip registration form, click here.
For more information on the Pexip software system and how-to guides, click here
Emergency Licensure Provisions Bylaw Amendments
New Bylaw Amendments Provide Expedited Emergency Registration/Licensure
The amendments ensure expedited, no-fee, temporary emergency licensure/registration for Retired, Associate, Non-Practising members, pharmacy professionals who were previously registered/licensed with the SCPP, and pharmacy professionals (in good standing) from other Canadian jurisdictions. These professionals must have been previously licensed/registered within the last three years, be in good standing, and have no ongoing complaints investigation or disciplinary findings.
These amendments may help to address potential Pharmacist or Pharmacy Technician shortages resulting from the COVID-19 pandemic (or any other potential future emergencies). Please see the Emergency Registration and Licensure – Supplemental Policy for more information.
A pre-registration screening form is provided for further information and to express interest in obtaining this licence. Please also visit the Emergency Registration section for more information.
Amendments to Provide Interns with the Authority to Complete Advanced Method Certification (AMC) in an Emergency
At the discretion of the Registrar, this new authority will ensure an optimal number of pharmacy professionals are available to administer the flu vaccination, a potential COVID-19 vaccination, or other necessary medications by injection or other routes. This exemption for AMC is in place for 2020 graduates only as part of the emergency licensure bylaws.
Q-and-A on the Workplace, Closures and Staffing
These public health measures are effective April 9, 2020 but may change over time – please see the Saskatchewan government website for current information for businesses and employees.
For additional information on cleaning and infection control, see the below resources:
- medSask – guidelines and recommendations for appropriate cleaning and disinfection of surfaces in community pharmacies during the COVID-19 pandemic?
- SCPP Reference Manual – Infection Control Guidelines
- SCPP Reference Manual – Respiratory Hygiene and Cough Etiquette Standards and Guidelines
- SCPP Reference Manual – Hand Hygiene
New Infographic Poster for Pharmacies and Physicians
Update - Modifying Pharmacy Hours Policy & Procedure
Privacy Concerns – Reporting Patients Not Complying with Legal Requirement to Quarantine
- SCPP has been receiving inquiries from pharmacy team members regarding the reporting of patients to the police who are not complying with the new legal requirement (both Provincial and Federal under the Quarantine Act announced March 26, 2020) to quarantine themselves whether it be after a trip outside of Canada regardless of showing symptoms or when they are experiencing symptoms of COVID-19.
- The section below from the Office of the Saskatchewan Information and Privacy Commissioner (OIPC) speaks to the authority to disclose this information to law enforcement individuals. In addition, the privacy officer for each pharmacy should be familiar with the privacy documents on SCPPs website, specifically the Disclosure of Personal Health Information to Law Enforcement Authorities Guidelines for Pharmacists and Pharmacy Technicians, that speaks to disclosing information to avoid or minimize danger to the health and safety of any person.
IPC Guide to HIPA
27(4) A trustee may disclose personal health information in the custody or control of the trustee without the consent of the subject individual in the following cases:
(a) where the trustee believes, on reasonable grounds, that the disclosure will avoid or minimize a danger to the health or safety of any person;
Disclosure of Personal Health Information to Law Enforcement Authorities Guidelines for Pharmacists and Pharmacy Technicians
“Where, in the reasonable opinion of the member, the disclosure of personal health information is required to avoid or minimize a danger to the health or safety of the client, staff or any third party, the member may disclose the information without the consent of the client.”
Use of PAR and Modified Notification to Patient's Provider
Transferring CDSA Drugs in Software
Modifying Pharmacy Hours Policy & Procedure
Requirements for Chain of Signatures in Light of COVID-19
Shortage of Hand Sanitizer
- In response to the shortage of hand sanitizer, Health Canada has advised that in response to the unprecedented demand and urgent need for disinfectants and hand sanitizers during the COVID-19 pandemic, it is excluding this bulk compounding from the requirements of the Food and Drug Regulations and the Natural Health Products Regulations within the context of the practitioner-patient relationship. Retail sale to the public likely does not satisfy this requirement and would require Health Canada authorization prior to the sale. However, should pharmacies choose to compound hand sanitizer, and make it available to the public (outside of the practitioner-patient relationship), Health Canada will not prioritize the enforcement of this condition, on the condition that:
- It be compounded by a licensed pharmacist within Saskatchewan; and
- It be created following a reputable formula such as that published by the World Health Organization (e.g. Guide to Local Production: WHO-recommended Handrub Formulations).
The SCPP supports the compounding of hand sanitizer to meet patient and professional practice needs at this time with additional expectations:- It must be done by a licensed pharmacist or licensed pharmacy technician working with a pharmacist;
- Professional ethics of pharmacy professionals would be maintained at all times; and
- Once the shortage is resolved, all commercial manufacturing would cease.
- Ongoing meetings between the SCPP and the Ministry of Health continue. The Ministry of Health is aware of the shortage of personal protective equipment for community pharmacies and the public demand for medication stockpiling which could lead to drug shortages. The Ministry is following up on these concerns. The Pharmacy Association of Saskatchewan is working with its members to gather PPE information. Updates will be provided as they become available.
- Thank you to those pharmacists who are educating the public about the risk of drug shortages due to unnecessary stockpiling. The Canadian Pharmacist Association has developed key messages to help explain the risks of medication stockpiling to the public.
New Emergency Preparedness Tools Document
- A new Emergency Preparedness Tools document has been prepared by SCPP to inform pharmacy professionals of the tools available to help them address the risks they may be identifying in their preparations to address the COVID-19 pandemic.
Document: Emergency Preparedness Tools (updated March 18, 2020)
The College is working closely with the federal and provincial ministries of health, regulators and other health system partners to undertake the necessary planning to protect residents. - SCPP met with Health Canada and the College of Physicians and Surgeons to discuss emergency planning measures for an exemption to allow pharmacists to prescribe narcotics and controlled drugs in Saskatchewan should it be needed.
- SCPP met with representatives from national and provincial pharmacy associations and regulators to provide updates and discuss areas for national action.
Provincial and Federal Announcements
COVID-19 Program Update – mRNA Bivalent Vaccines for Primary Immunization Series for All Ages as of July 24, 2023
- Monovalent mRNA vaccines will no longer be available for primary series for all ages as of July 24, 2023.
- All monovalent mRNA vaccines should be disposed of by July 23, 2023.
- Age-appropriate bivalent Omicron-containing vaccines should be administered to start or complete a COVID-19 vaccine primary series in those 6 months and older.
Extended: Non-Traditional Immunizers
The Ministry of Health has extended the approval of non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns, and pharmacist extended interns to administer publicly-funded COVID-19 and Influenza vaccines.
Please see the Ministerial Order Designating Additional Vaccine Providers for COVID-19 and Influenza Vaccine here.
These immunizers are enabled by provisions in The Public Health Act, 1994 and through SCPP emergency bylaws and are valid until March 31, 2024.
Note that only pharmacy student interns, extended interns and pharmacy technicians remain as non-traditional immunizers able to administer COVID-19 and influenza vaccines. Effective 11:59 pm on March 31, 2023, all other emergency immunizers can no longer provide immunizations unless their normal scope of practice enables it.
The following applicable documents and web page will be updated shortly:
COVID-19 Booster Dose Clarification and Interval Revision
Expired/Expiring Vaccine
The below was sent to pharmacy managers by the Drug Plan and Extended Benefits Branch.
Expiring Dec. 8, 2022:
| Vaccine | DIN | LOT | EXPIRY |
| Moderna Monovalent (red cap/light blue label) | 02510014 | 069B22A | December 8, 2022 (MUST be discarded after today) |
Expired vaccines still appearing in community pharmacy VDTS accounts:
| Vaccine | DIN | LOT | EXPIRY |
| Pfizer-BioNTech (purple cap/ purple boarder) | 02509210 | FN7934 35035BD FM2952 FM7380 FN9501 FT7276 | 2022-Oct-31 2022-Oct-31 2022-Oct-31 2022-Nov-30 2022-Nov-30 2022-Nov-30 |
Please ensure your fridges are checked, expired vaccine is discarded and inventory is updated via VDTS and submit a wastage report to the Ministry.
Cold Chain Processes
In the month of November the Ministry of Health noted an increase in the number of pharmacies submitting cold chain break reports due to human error. The fall season is a very busy time, however, it is essential that the utmost care be taken in the storage and handling of all biological products, in order to minimize wastage and protect the potency of the vaccine.
Please review the following important vaccine storage principles:
- Place new stock into the refrigerator immediately after receiving it from your distribution center.
- Keep vaccines in the refrigerator or in a cooler with cold packs until ready to administer.
- Keep all vaccines in their original boxes until they are ready to be used (protect from light, if applicable)
- Never leave vaccine outside the refrigerator (e.g., on counters or in coolers after finishing a clinic or at the end of day).
- Carefully scan you work environment frequently and at the end of each day to prevent cold chain breaks due to human error.
Thank-you for your ongoing support of the COVID-19 and Influenza Immunization Programs in Saskatchewan.
Paxlovid – Expiry Extension to 18 months
Health Canada has issued a Notice of Compliance (NOC) for the shelf-life extension of PAXLOVID (nirmatrelvir and ritonavir) from 12 months to 18 months for multiple LOT#s of the Standard Dose Pack (DIN: 02524031) and the Moderate Renal Impairment Dose Pack (DIN: 02527804). Pharmacy managers were sent the below information:
- Blisters and cartons with an expiry date of August 2022 printed on the label may remain in use for an additional 9 months beyond the printed date.
- Blisters and cartons with an expiry date of January 2023 through May 2023 printed on the label may remain in use for an additional 6 months beyond the printed date.
- The approved storage conditions remain unchanged.
- The expiration date of Paxlovid Tablets must be verified prior to dispensing.
As required by the NAPRA Standards of Practice (see 1.3 and 3.1 here), pharmacy professionals must verify the accuracy of dispensed prescriptions and communicate with patients effectively. Pharmacy professionals must consider the following actions when dispensing the impacted lot# of Paxlovid®:
- Blacking out the expiry dates on the external box and each blister card, and applying a label showing the new expiration date;
- Verifying the expiration date prior to dispensing; and
- Reviewing the modifications with the patient to reassure them it is safe to use.
Affected LOT#s and extended expiry dates:
Please see the Pfizer letter for complete details.
If you have any questions, please contact DPEBImmunizations@health.gov.sk.ca.
Bivalent Booster Fall Program
Expansion of Moderna Bilvalent Dose Eligibility
Provision of Moderna Spikevax® Bivalent Original/Omicron Vaccine
Vaccine Boosters for Children 5-11 Years Old
Expansion of Booster Dose Eligibility
Pharmacists Prescribing Paxlovid
The Ministry of Health is expanding access to Paxlovid by increasing the eligibility criteria and expanding who may prescribe for this publicly funded drug. The Paxlovid Distribution, Prescribing and Assessment program officially launches on May 19, 2022 and is in effect until Oct. 31, 2022 or at the discretion of the Ministry of Health and/or the Chief Medical Health Officer.
The expanded prescribers include community physicians, nurse practitioners and pharmacists. SCPP Council met on May 2, 2022, and approved pharmacists prescribing for Paxlovid for the treatment of mild to moderate COVID-19 with the following conditions.
Pharmacists who prescribe Paxlovid will be required to:
- receive training offered by CPDPP prior to prescribing – training occurred on May 12 and 13 and the sessions were recorded for ease of access.
- follow the medSask algorithms, guidelines, patient assessment record and drug interaction tools as approved by Council, and
- follow any other conditions specified by the Ministry of Health and/or Saskatchewan Health Authority for this publicly funded program.
Please see the medSask website for access to the following documents:
- Paxlovid Patient Assessment Record (PAR)
- Paxlovid Algorithm
- Paxlovid Eligibility Overview
- Paxlovid Drug Interactions Table
- Paxlovid Patient Handout
To assist members, please also see SCPP's Paxlovid Prescribing – Frequently Asked Questions for Pharmacists.
Clarification on COVID-19 Booster Dose Intervals
Clarification on COVID-19 Booster Dose Intervals
Expansion of COVID-19 Second Booster Eligibility
Update: Non-Traditional Immunizers
The Ministry of Health has extended the approval of non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns, pharmacist extended interns, and formerly licensed pharmacists, to administer publicly-funded COVID-19 and Influenza vaccines.
Please see the Minister's Order Designating Additional Vaccine Providers for COVID-19 and Influenza Vaccine here.
These immunizers are enabled by provisions in The Public Health Act, 1994 and through SCPP emergency bylaws and are valid until March 31, 2023.
Please see the documents below for detailed guidance:
- All individuals 70 years of age and older living in Saskatchewan.
- All individuals 50 years of age and older living in First Nations.
- All individuals 50 years of age and older living in the Northern Service Administration District (NSAD) – Far North in Saskatchewan.
- Healthy individuals aged 12 years of age and older (interval applies to first booster doses).
- Adults living in a long term care home or other congregate living setting for seniors 65 years and older (interval applies to first and second booster doses).
Note the current authorizations as outlined in Saskatchewan's Chief Medical Health Officer's letter here.
COVID-19 Booster Doses for 12-17
Please see the notice here.
Amendment to COVID-19 Vaccine Booster Dose Program
Additional COVID-19 Vaccine Booster Doses to Select Populations

Additional COVID-19 Immunizations Discontinued for Travel Purposes
Saskatchewan Expands Booster Dose Program
The Government of Saskatchewan continues with booster dose expansion.
Effective December 20, 2021, all individuals 18+ will be eligible for a booster shot three months out from their second dose.
Vaccines are widely accessible through clinics and pharmacies in communities across the province. To date, the uptake of booster and third doses for all eligible residents is 38 per cent, with more than 159,649 administered, see the full announcement here
COVID-19 Vaccine Expanded Booster Information
The Government of Saskatchewan continues with booster dose expansion.
Effective immediately, the following individuals are eligible to receive third/booster doses as noted below, see the full announcement here
Populations Eligible for Booster Doses Immediately:
- Individuals aged 50 years and older
- All health care workers
- Individuals 18 years and older living in the far north and those living on First Nation communities
- Individuals born in 2009 or earlier with underlying health conditions that are clinically extremely vulnerable, including those with diabetes
Effective immediately for all booster target populations the recommended interval between second doses and booster doses is five months instead of six months.
COVID-19 Vaccine Booster Information and Additional Information
The Government of Saskatchewan has provided information on booster dose expansion and clarification, three dose primary series for immune compromised individuals, and pediatric vaccine clarification.
Effective immediately, the following individuals are eligible to receive third/booster doses as noted below, see the full announcement here
Those living in:
Immunizations for Five- to 11-year-olds
The Ministry of Health has advised that with the Health Canada approval of the Pfizer COVID-19 vaccine for children aged five- to 11-years-old, immunizations for this age group will begin. See the announcement here.
Vaccinations for five- to 11-year-olds will be available at a wide variety of locations including participating pharmacies, Saskatchewan Health Authority (SHA) walk-in clinics, mobile clinics, at schools, and at venues with easy community access near schools.
The Pfizer pediatric vaccine is a slightly different formulation with smaller doses of vaccine and immunizers will need to complete the applicable SHA training online to ensure they are competent to provide pediatric immunizations.
Please see the following helpful resources:
Update of Section 56 Exemption
Please see the applicable updated documents below:
Johnson & Johnson Vaccinations Available at SHA Clinics
The Government of Saskatchewan issued a news release announcing that a limited supply of Janssen COVID-19 vaccines will arrive in the province for administration through a number of Saskatchewan Health Authority (SHA) clinics, effective Nov. 17, 2021.
See the news release here.
- Recipients of the single-dose Janssen vaccine are eligible for an mRNA vaccine booster dose two months after their vaccine (for all recipients, 18 years and older).
- Pharmacies can administer either Pfizer or Moderna to patients two months after their single dose of Janssen vaccine.
Non-Traditional Immunizers for Influenza Immunization Program
The Ministry of Health has approved non-traditional immunizers including licensed pharmacy technicians, pharmacist student interns and extended interns, and formerly licensed pharmacists, to administer publicly-funded influenza vaccines. Please see the Order of Council notice here. For the complete list of non-traditional Influenza immunizers, please click here.
These immunizers are enabled by the emergency provisions to the Disease Control Regulations and through SCPP emergency bylaws and are valid until March 31, 2022.
Please see the updated Influenza Season Preparation poster as well as the Immunizer FAQs.
Q-and-A Booster Shots for Health Care Workers
The below was communicated by the Drug Plan and Extended Benefits Branch on Oct. 21, 2021.
The following Q-and-As provide further clarification on the eligibility of health care workers for a COVID-19 vaccine booster dose effective Oct. 25, 2021.
Q: What identification will health care workers be required to provide in order to receive their boosters?
Health care workers will need to present one of the following: a copy of their licence from their professional licensing body, OR a workplace pay stub at the point of immunization OR, if they are an SHA employee, their Saskatchewan Health Authority staff identification to demonstrate that they are health care providers. It’s vital that they are at least six months from their second dose, so checking their vaccination record is key for eligibility.
Q: Is it all health care workers? In previous immunization roll-out phases, we were provided a list of occupation types.
YES, this is for ALL health care workers regardless of position. Proof of employment OR the credentials of a licensing body, in addition to being six months from their second dose, will be the only proofs required to qualify for a third dose at this time. Cross-referencing workers by occupation and determining the type of documentation that would be required has been identified as a barrier to immunization.
Please see the directive from Saskatchewan's Chief Medical Health Officer.
Expanding Booster Dose Eligibility
Starting Oct. 25, 2021, the COVID-19 vaccination booster program will be expanded. COVID-19 boosters will be administered at least six months after the second dose was received for the following groups:
- Individuals aged 65 years and older.
- Individuals living in the Far North and those living on First Nation communities, aged 50 years and older.
- Health care workers, who will be asked to present a copy of their license from their professional licensing body or a workplace pay stub at the point of immunization. Saskatchewan Health Authority staff will be required to present their staff identification.
- Individuals born in 2009 or earlier with underlying health conditions that are clinically extremely vulnerable including:
- People with severe respiratory conditions including all cystic fibrosis, severe asthma, and severe chronic obstructive pulmonary disease (COPD)
- People with rare diseases that significantly increase the risk of infections, such as homozygous sickle cell disease
- People who had their spleen removed
- Adults with very significant developmental disabilities that increase risk, such as Down's Syndrome
- Adults on dialysis or with chronic kidney disease (stage 5)
- Significant neuromuscular conditions requiring respiratory support
For more details, see the news release.
Joint Statement - Section 56 Exemption Extension
Acceptable Proof of Vaccination
As per the recent news release from the Ministry of Health, immunization records printed by the pharmacy from the eHR Viewer are not accepted as valid proof of vaccination.
The following are the accepted options for proof of vaccination.
- QR code/MySaskHealthRecord vaccine certificate either printed or on the patron's mobile device as a screenshot or in SK Vax Wallet;
- A printed hard copy of MySaskHealthRecord vaccine certificate with or without a QR code;
- Wallet cards issued at the time of vaccinations; and
- A COVID-19 vaccination printout from Saskatchewan Health Authority (SHA) public health.
See the media release for more details.
COVID-19 Vaccine Contraindications Document Updated
Expansion of COVID Self-Testing
Expanded Booster Dose Eligibility
A complete list of eligible individuals is available at Saskatchewan.ca/covid-19.
If a pharmacy wishes to increase its COVID-19 vaccine allocation for the week of Oct. 4 (delivery on Oct. 6), they need to submit those requests by 12:00 pm, Wednesday, Sept. 29 by email to DPEBimmunizations@health.gov.sk.ca.
Vaccine Brand Updates
Automated COVID-19 test results available in Saskatchewan
With the increase of positive COVID-19 cases across the province, the Saskatchewan Health Authority has moved all initial notifications for COVID test results to an automated service. In May 2021, the SHA launched the auto-notification for negative COVID-19 test results. Now, beginning on September 17, anyone tested for COVID-19 in Saskatchewan will be quickly informed of both their positive and negative COVID-19 lab test results through text message or voice call message notifications.
For more information about automated COVID-19 test results, please visit saskatchewan.ca/COVID19. For the full announcement, please click here.
Provision of COVID-19 Vaccine Booster Doses to Select Populations
.png)
Moderna Authorized for Children Born 2009 and Earlier
Children should receive the same mRNA vaccine brand for their COVID‐19 vaccine series. It is important that immunizers double-check the immunization records of children presenting for COVID‐19 immunization to avoid giving a mixed vaccine brand series to children if possible.
See the letter of authorization from Saskatchewan's Chief Medical Health Officer.
Intervals Between COVID‐19 and Other Vaccines No Longer Required
COVID-19 Vaccination Boosters Starting Sept. 7
Details about the campaign include:
- Those who will be eligible include residents of long-term care and personal care homes; stem cell and solid organ transplant recipients; recipients on stable, active treatment (chemotherapy, targeted therapies, immunotherapy) for malignant, hematologic disorders; recipients of an anti-CD20 agent (e.g. rituximab, ocrelizumab, ofatumumab).
- Letters will be sent out to those who are eligible this week either from their physician or from the Ministry of Health.
- The required interval for a booster dose must be at least 28 days after receipt of the second mRNA dose.
- Details on the additional phases will be available soon.
- Evidence indicates that factors that contribute to the waning of the effectiveness of COVID-19 vaccines include the age of the recipients (elderly waning more quickly) and if the individual is immunocompromised due to a medical condition or medication.
Pfizer Extended Expiry for Vaccine in ULT
The Ministry of Health has advised that Pfizer-BioNTech has extended the shelf life only for vaccine stored at ULT temperatures by three months. More information below:
- As per this notification, Pfizer-BioNTech has extended the ULT shelf-life of their COVID-19 vaccine by three (3) months.
- This includes product already in country, and product that will be arriving in the future.
- Expiry dates on all packaging, from August 2021 to February 2022, is extended by three (3) months (see table below).
- All sites will have to keep track of the updated expiry dates themselves.
- The manufacturer will not be updating their labels.
- The extended shelf life only applied to vaccine stored at ULT temperatures. The product monograph remains unchanged related to storage/transport at frozen (-20*C), fridge (2 to 8*C), and room temperature (up to 25*C)
- Pfizer-BioNTech has also extended the ULT temperature storage parameters.
- Vials can now be stored/transported between -90C and -60C.
- The Ministry’s cold chain work standard will be updated as soon as possible to reflect the changes.
- The product monograph at cvdvaccine.ca will be updated shortly - see table below from page 22.
| Printed Expiry Date |
| Updated Expiry Date |
| August 2021 | > | November 2021 |
| September 2021 | > | December 2021 |
| October 2021 | > | January 2022 |
| November 2021 | > | February 2022 |
| December 2021 | > | March 2022 |
| January 2022 | > | April 2022 |
| February 2022 | > | May 2022 |
Vaccines for Ages 12-17
While Health Canada has approved the Moderna COVID-19 vaccine for children aged 12 and over, immunizers in Saskatchewan are not authorized to administer Moderna to those aged 12-17 until authorization is provided by the Ministry of Health. Please check the COVID-19 page as well as the SCPP newsfeed regularly for any updates.
Pfizer Vaccine Approved for Children Born in 2009
Additional Doses Of COVID-19 Vaccine Approved For Travel
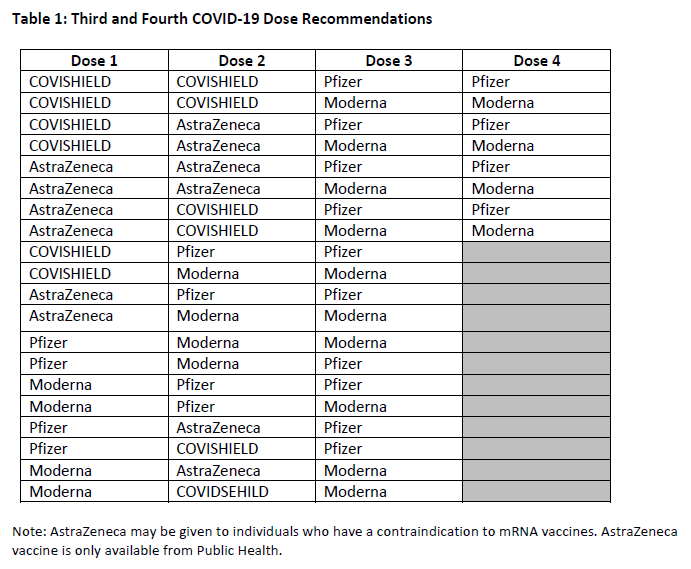
Resources
Daily Practice
- Community Pharmacy Practice Enactments
- COVID-19 Immunizers FAQs
- Status Update – COVID-19 Rapid Antigen Tests in Pharmacies
- Section 56 Exemption Communication
- Electronic Transmission and Storage of Prescriptions – COVID-19 Exemptions
Emergency Preparedness
- Emergency Preparedness Tools
- Emergency Preparedness Resource Kit
- Ethical Duty during an Emergency, Disaster or Pandemic
- Emergency Preparedness – Modifications to Pharmacy Operations and Hours
- Modifying Pharmacy Hours of Operations Procedures
- Pharmacy Closures (Temporary): Model Regulatory Policy Due to Pharmacist Absence
Infection Control
Prescriptive Authority
- Paxlovid Prescribing – FAQs for Pharmacists
- Emergency Exemptions for Prescribing Authority
- Developing a Safe and Functional Collaborative Practice Agreement
Dispensing
- COVID-19 Immunization Program
- COVID-19 Immunization Manual
- Temperature Log for Vaccines
- COVID-19 Vaccine Page
- Public Health Requirements for COVID-19 in Community Pharmacies
- COVID-19 Self-Testing
- Pharmacy Poster - Protect Yourself and Others - Stop the Spread of Viruses
- COVID-19 Travel Information
- COVID-19 Self-Monitoring Information
- COVID-19 Self-Isolation Information
For more information, please see saskatchewan.ca/coronavirus.
For more information, please see saskhealthauthority.ca.
For more information, please see https://medsask.usask.ca/.